Pathological pattern recognition: AI uses animal data
Animal samples also help humans to detect cancer using AI in pathology – without the need for animal testing.
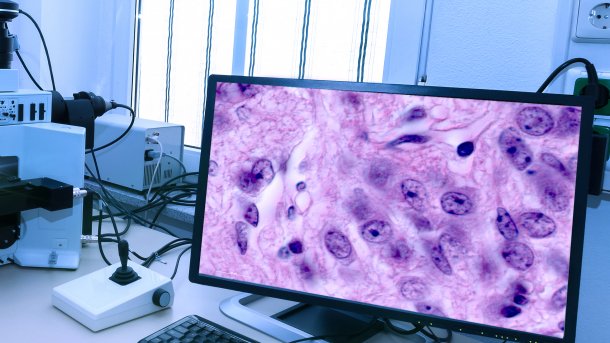
Pathology takes place not only in the laboratory or in the dissection room, but also on the screen, as tissue samples are digitized.
(Image: tilialucida/Shutterstock.com)
If you want to conduct research with AI, you need large quantities of quality-assured data. There are discussions about where medical research data comes from, who is allowed to access health data in the central data portal, how health data and data protection are handled in general and who owns the health data. It does not always have to be people who provide data for medical research. Animal data can also be used in pathology, for example, to train an AI to detect cancer in animals and humans – without the need for animal testing.
Inter-species transfer between animals and humans
Research is not always dependent on primary data that is collected specifically and actively for studies. Research can also be carried out with secondary data that is collected as routine data during diagnostics and care. Professor Marc Aubreville has been working on the topic of algorithmically supported pathology since 2016 and conducts research on this topic at Ingolstadt University of Applied Sciences. He and other cooperation partners only use routine diagnostic data to train AI models for histopathology. The majority of this data does not come from humans, but from the medical treatment of pets such as dogs and cats.
Although animals and humans are different, there are parallels at cellular level: "Ultimately, our research should benefit living beings, so it is good to work with representative data for this application", says Aubreville. Where there is a high degree of biological parallelism, results can be transferred from animals to humans and vice versa and used in practical applications. Studies have shown that AI models trained on animal data can also achieve good results on human tissue. For example, an algorithm trained on canine breast cancer to recognize cell divisions could also be successfully applied to human breast cancer tissue.
Pathological truth based on pattern recognition
AI can not only detect cancer in radiology, but can also be helpful in other imaging procedures. In histopathology, the reliable diagnosis of tumor diseases takes place, in which tissue and cell samples are examined in detail under the microscope in the form of prepared tissue sections. The pathologist looks for abnormalities and patterns at cellular level. The diagnosis is based on pattern recognition. This is because certain patterns stand for certain pathologies.
Videos by heise
If the pathologist recognizes a pattern, he can draw conclusions about a disease. The distribution and concentration of pathological cells also allows conclusions to be drawn about the nature of the tumor, its course and prognosis. The analysis of a sample can take several days to weeks. If cancer is suspected, the pathologist evaluates the mitosis activities at cell level. Mitosis is a process of cell division. If a cell is in the process of cell division, this is referred to as a myototic figure (MF).
If the process is dysregulated, cells can grow and multiply uncontrollably, leading to the development and spread of tumors. Cancer cells are often characterized by an increased proliferation rate, i.e. they divide faster and more frequently than normal cells. This is reflected in an increased number of MF in the tissue. Pathologists have different methods to assess mitotic activity. They count the MF as Mitotic Count (MC) and determine the ratio of MF to the other cells that are not in mitosis as Mitotic Index (MI).
In order to better assess the aggressiveness of the tumor, they use the Volume Corrected Mitotic Index (M/V Index) to determine the number of MFs per unit volume of tumor tissue. In addition to normal MFs, atypical MFs are less common and indicate a more aggressive tumor biology. The identification and quantification of MFs can be difficult due to their complex morphology and can lead to divergent assessments between pathologists in the search for pathologic truth in the sample.
Digital pathology needs robust algorithms
Pathology is largely a manual process and an experiential profession. The evaluation of a histopathological specimen searching for cells suspected of being cancerous, i.e. the MF, can be imagined as the search for hydrants in a satellite image with a very high resolution, explains Aubreville. It is a very time-consuming process for humans, which also requires concentration. AI can help pathologists to make their diagnoses faster, more accurately and more efficiently. To do this, the tissue sections fixed on a slide must first be digitized by a Whole Slide Image Scanner (WSIS). Algorithms can be trained to automatically recognize and count the MFs in the digitized tissue sections (Whole Slide Image, WSI) and quickly visualize their distribution and patterns for the pathologist.
Such a WSI of a tissue sample for research can have a resolution of 300,000 pixels × 300,000 pixels. It takes several months of work to annotate a single WSI completely and correctly. To increase the accuracy of the annotations and reduce errors, a WSI is not annotated and checked by one but by several pathologists.
Empfohlener redaktioneller Inhalt
Mit Ihrer Zustimmung wird hier ein externes YouTube-Video (Google Ireland Limited) geladen.
Ich bin damit einverstanden, dass mir externe Inhalte angezeigt werden. Damit können personenbezogene Daten an Drittplattformen (Google Ireland Limited) übermittelt werden. Mehr dazu in unserer Datenschutzerklärung.
Apart from the annotation, the quality of the material is also a challenge for AI. Although there are standards, there are many influencing factors, from sample collection to sample preparation to the finished scan. Deviations occur in the form of the variability of tissue types and sections, staining methods and also the scanners used. "On the one hand, we are developing AI algorithms that are inherently more robust and, on the other, we are increasing sample heterogeneity to achieve a realistic reflection of practice," says Aubreville. Otherwise, any domain shift that leads to variations in the training material during the process from the sample to the digital image can also lead to a drastic drop in the detection rate of the AI in practice.
International exchange of animal data
To work with diagnostically collected samples and data from humans, researchers not only need their consent, but also the approval of an ethics committee. Animal samples are currently easier to obtain and handle for research, as consent and an ethics application are required again. With animal data, there is a larger and more diverse database. The Research Group of the AIMI Lab – Artificial Intelligence in Medical Imaging at Friedrich-Alexander University (FAU) and The Ingolstadt Medical Imaging Group at Ingolstadt University of Applied Sciences make this data freely available to other researchers on deepmicroscopy.org. To create an overview of biomedical AI models for research purposes, FAU has set up the"AIMe-Register" as a community-driven reporting platform.
Internationally, there are not only projects, but also medical image analysis challenges. As part of a Mitosis Domain Generalization Challenge (MIDOG), Aubreville and other participating research partners developed a mitosis detector in 2021, which is currently being tested as an additional diagnostic aid at the University Medical Center Utrecht.
(dahe)