eHealth: Coding and AI in gastroenterology, where the breakthrough is missing
Digital methods also play a role in gastroenterology. We spoke to Professor Hann about what is possible and where the limits lie.
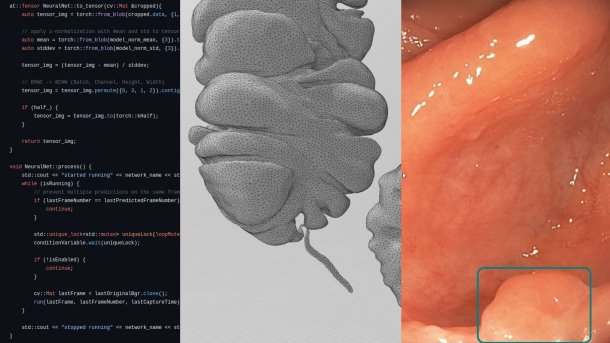
Code can be seen on the left, next to it a 3D intestine for VR simulation and an AI-detected polyp.
(Image: Alexander Hann, Universitätsklinikum Würzburg)
AI and VR are also playing an increasing role in digital medicine; simulations can help prepare patients for a serious operation in the operating room, for example. We spoke to Alexander Hann about what is possible in the field of gastroenterology and where the limits lie so far.
(Image: Daniel Peter)
Hann has been Professor of Digital Transformation in Gastroenterology at the University Hospital of Würzburg since March 2023.
What hopes are there for AI in colorectal cancer screening?
Colorectal cancer is one of the most common cancers in men and women – both in Germany and worldwide. The special thing about it: This type of cancer can be prevented as it develops via easily recognizable precursors, so-called adenomas. Adenomas are polyps, i.e., mucosal growths in which cells increase in size.
(Image: Alexander Hann, Uniklinikum Würzburg)
Small polyps can develop into larger ones over the years and, on average, cancer develops after around ten years – usually between the ages of 60 and 65. This is why screening is so important from the age of 50.
Since when has AI been approved for colorectal cancer screening, and what are the hurdles to widespread use?
AI has been used in colorectal cancer screening since around 2019. All major manufacturers now offer corresponding systems. This makes gastroenterology one of the first disciplines to use deep learning models based on large image data sets. However, this does not mean that the technology is used everywhere. The AI recognizes polyps and marks them with a so-called bounding box during the examination. These systems are known as CADe (Computer Assisted Detection Systems) and support endoscopic examinations.
Do such systems also exist in other areas of medicine?
Yes, for example, CADe systems are used in mammography for the early detection of breast cancer as a second opinion for radiologists. They also help to detect metastases in prostate or lung cancer at an early stage. The US FDA maintains a list of approved AI-based medical devices – most of which come from radiology or cardiology. However, most randomized studies have so far been conducted in gastroenterology.
So, have the systems in gastroenterology been particularly thoroughly researched?
Absolutely. There are many approved AI applications, but more than 40 randomized clinical trials have already been conducted in gastroenterology, with more than 30,000 patients examined worldwide.
The use of AI has significantly increased the detection of precancerous lesions in studies – by an average of 8 percentage points. This is encouraging and corresponds exactly to what I would like to see as an endoscopy user: I can find more with AI. However, the first studies showed a stronger effect than more recent data.
Statisticians from the British Medical Journal (BMJ) evaluated 40 randomized studies in a meta-analysis and asked themselves: What are the relevant endpoints for those affected? Whether I find a few more or fewer precursors does not in itself bring any direct benefit for patients.
Why is that the case?
The existing randomized clinical trials on colorectal cancer screening do not usually investigate how many cases of cancer are actually prevented or whether people live longer as a result. Long-term studies would be necessary to answer these questions – but the technology has only been available since 2019/2020.
However, we know from over 20 years of research that the number of adenomas (polyps as precursors of bowel cancer) in the bowel correlates with the later occurrence of cancer and with the survival chances of those affected. The statisticians at the BMJ have developed a microsimulation model and shown that if AI is used in 10,000 patients over ten years, only two colorectal cancer-related deaths are prevented – a very small and statistically insignificant effect. The BMJ therefore recommends that AI should not be used routinely in this area.
The really relevant endpoints for patients – such as the prevention of deaths – are therefore much lower than hoped for. This is sobering. These data were presented to three professional societies, and none of them were able to identify a clearly positive effect. The European Society for Endoscopy (ESGE), for example, stated that patients who decide to have an endoscopy would like to use AI – in the hope of being one of the few who actually benefit. However, if more precancerous lesions are detected, this can lead to shorter intervals between check-ups and patients having to attend follow-up care more frequently. This means they have to attend appointments more frequently and adjust their daily routine. However, no serious side effects such as deaths or bleeding have been observed.
The American Gastroenterology Association (AGA) was also confronted with the data. After several consultations and a public comment period, it decided not to make a recommendation for or against the use of the technology –, a first, as guideline committees usually take a clear position.
Is there a risk of “de-skilling” through the use of AI?
Yes, that is possible. In one study, we showed videos with and without AI support and used eye tracking to analyze how the gaze and image analysis of the test subjects changed. It was found that the monitor image was scanned significantly less thoroughly for precancerous lesions when the AI support was switched on – users increasingly rely on the technology. This harbors the risk of certain skills being lost or not developing at all. However, there is little research on this so far and we need to be careful about drawing definitive conclusions.
How do the systems from the various manufacturers differ?
When selecting an AI system, it is of course also interesting to see how the systems develop in comparison and how they change as a result of updates. In another study, we were able to show that some systems react faster after updates, but become slower again after a change of detection mode. It would be nice if manufacturers would publish such data themselves.
In our eye-tracking study mentioned earlier, we also investigated how beginners and advanced users evaluate images. This showed that the machine recognizes polyps significantly faster than humans. However, when humans and machines work together, the machine's time advantage is lost as the human has to check every detection. False-positive detections by the AI mean that humans can no longer fully rely on the AI. It would make sense here to suppress “short indicators” to increase clarity. Human-machine interaction is an important research topic that still needs a lot of attention. University hospitals should take a critical look at these technologies.
How should such technologies be integrated into care?
I think it's very positive that there are several centers in Germany where members of the medical, computer science and engineering departments work together on projects. For example, we are currently developing an interactive portal for cooking recipes with the target group of patients who suffer from taste disorders during chemotherapy. With the help of a large language model and the support of nutritionists, we recommend recipes to patients that suit their taste disorders. The “Gustabor” project is funded by the Bavarian Center for Cancer Research (BZKF).
I am convinced that the future lies in interdisciplinary cooperation in which clinics and medical practices provide the necessary data.
Will the Health Data Utilization Act make the data more accessible?
From a scientific perspective, it is clear that if an AI is trained with data from its center and then encounters data from another center, its performance often drops significantly. Among other things, this is due to different image acquisition processors and data processing, but above all to the varying data quality. The development of high-performance AI is therefore dependent on a multi-center, preferably prospectively collected data set.
In practice, we currently proceed as follows: For the use of retrospective data from our center, we submit an ethics application in each center and conclude a data sharing agreement for multicenter surveys. For prospective data, we also obtain informed consent from all included patients before study inclusion.
Who benefits from your projects published under MIT license?
In addition to Germany, our software is downloaded worldwide, for example in the USA, Japan, and China. The largest endoscopy manufacturers come from Japan. We make our data available via the university hospital's research website.
One goal of our work is to automate structured reporting. Our AI recognizes various findings in the video feed in real time during the examination and suggests a structured report. We use neural networks that specialize in image recognition for this.
How does this work in practice?
For example, our polyp detection AI also recognizes the instruments inserted, including resection wounds, and uses them to document when a polyp has been removed. The software creates timestamps and generates an image report at the end.
You also reported on a game designer at the DMEA. What is it all about?
We are working on a third-party funded project, funded by the Federal Ministry of Education and Research (BMBF) to research alternative methods to animal testing, in which we are developing a simulator in virtual reality (VR) to practice endoscopic procedures without animal material. The focus here is on hemostasis for gastric and intestinal bleeding. We want to create this VR-based alternative with a game designer, an engineer, and a doctor. In 2019, for example, we also published a study on an intuitive zoom method using VR in the gastroenterology journal Gut.
Empfohlener redaktioneller Inhalt
Mit Ihrer Zustimmung wird hier ein externes Video (TargetVideo GmbH) geladen.
Ich bin damit einverstanden, dass mir externe Inhalte angezeigt werden. Damit können personenbezogene Daten an Drittplattformen (TargetVideo GmbH) übermittelt werden. Mehr dazu in unserer Datenschutzerklärung.
How can AI be used in teaching?
(Image: Universitätsklinikum Würzburg)
We use AI to generate artificial images of colorectal cancer precursors for teaching purposes – similar to an image generator. This allows us to provide targeted training material for education without having to use patient-related images. I am convinced that the targeted use of AI in teaching offers great advantages.
Who is funding your extensive research work?
Our projects are funded by foundations and public third-party donors, such as the Federal Ministry of Research, Technology and Space, the Bavarian Center for Cancer Research, the Wilhelm Sander Foundation, German Cancer Aid and, in particular, the Eva Mayr-Stihl Foundation. We also have important cooperation partners such as Stuttgart Hospital. If one of our self-developed AI systems is approved as a certified medical product, we will need an industrial partner. I would like to continue to concentrate on researching and developing prototypes with a direct clinical benefit with the working group and not take on any sales activities.
(mack)